Ground State Electron Configuration Chart
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
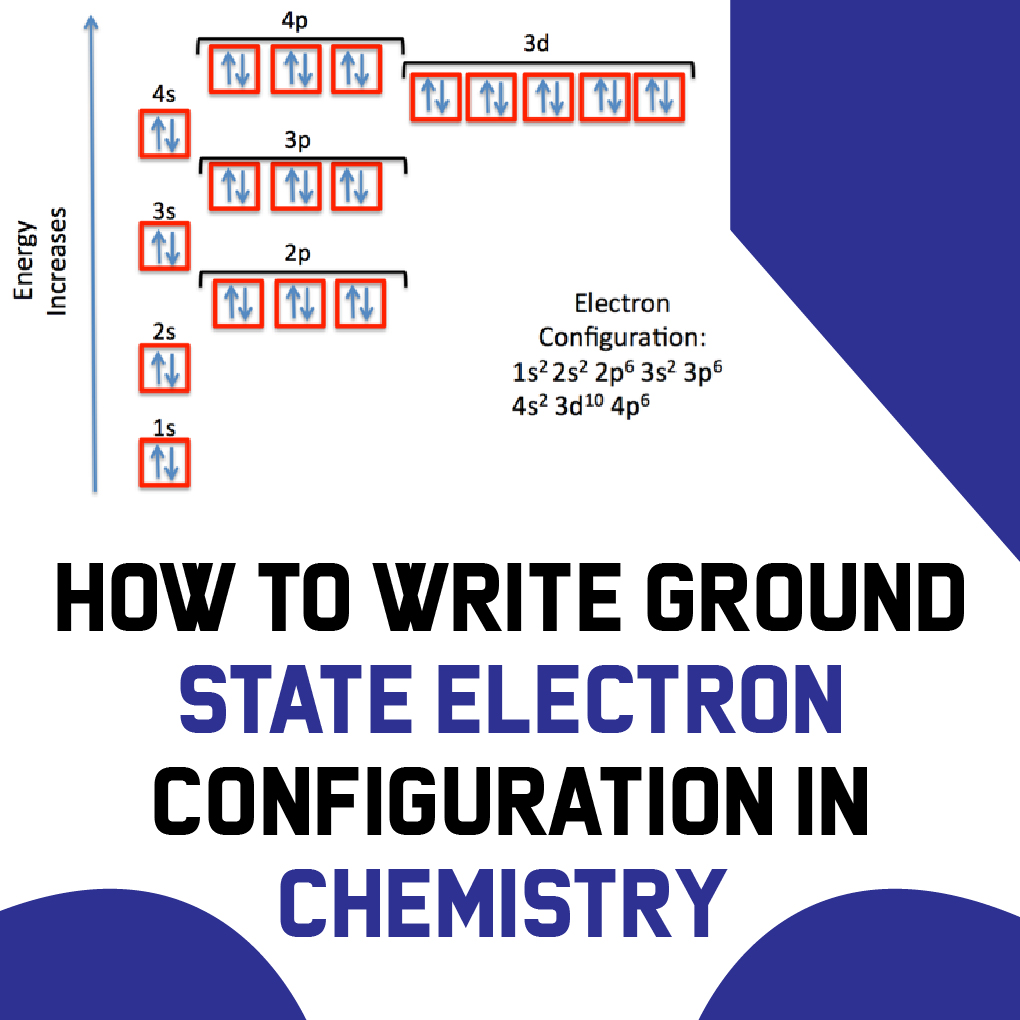
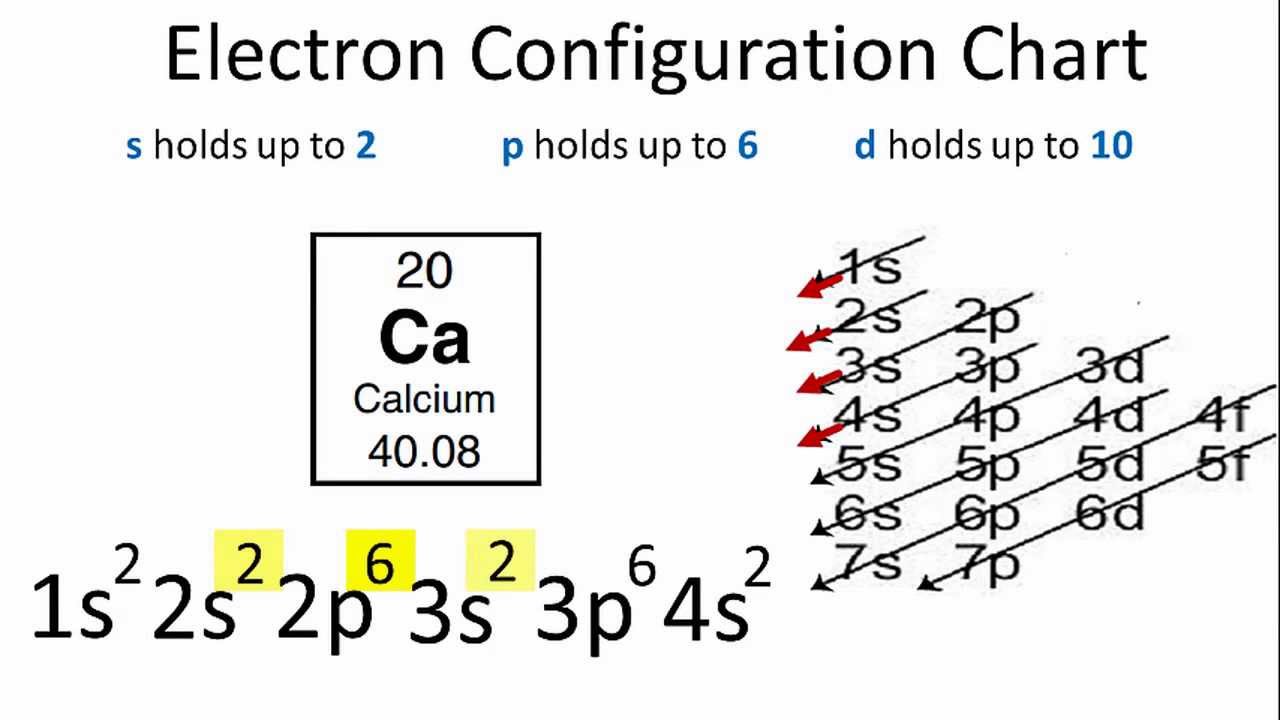
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2.
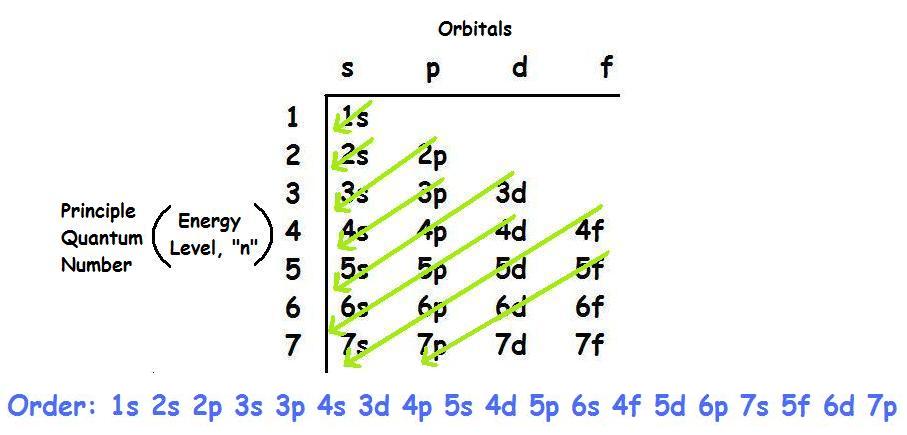
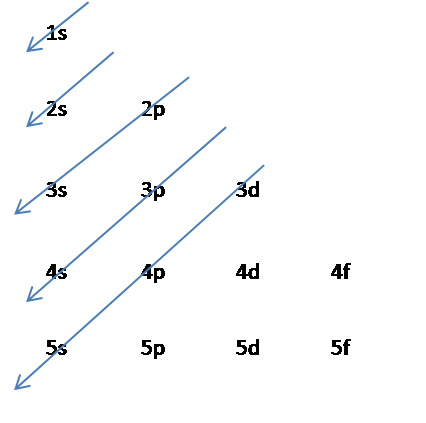
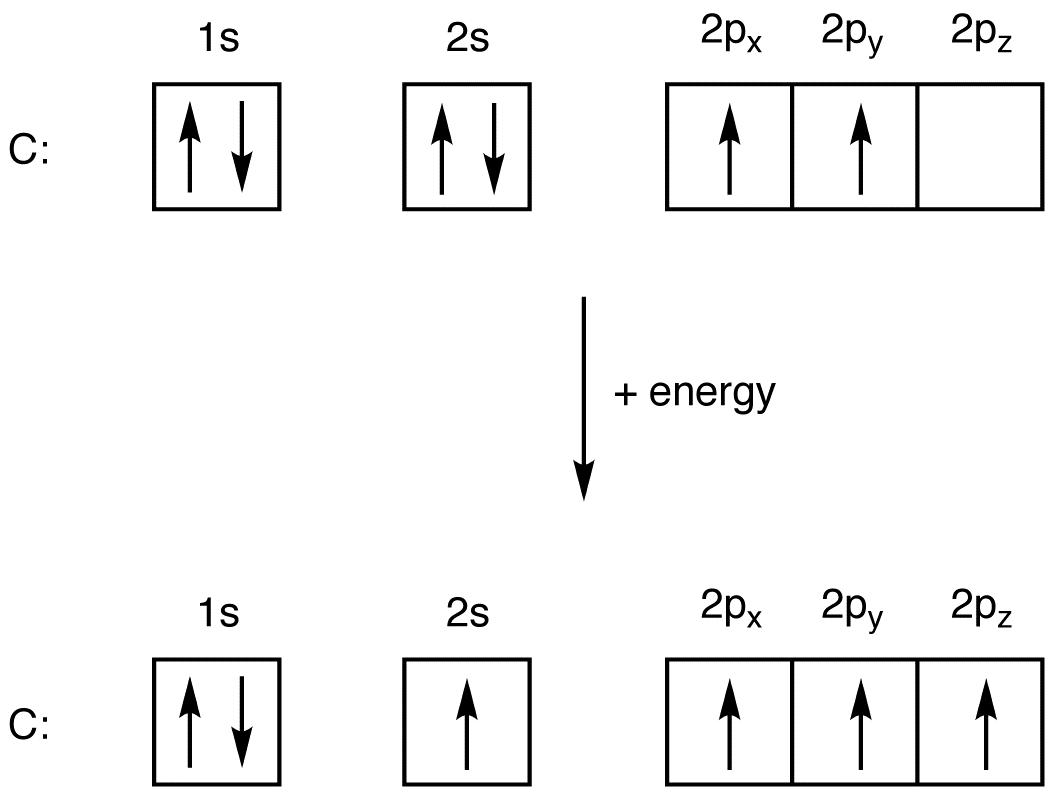
Ground state electron configuration chart. Identify the ground state using Hunds rule maximum multiplicitymaximum of parallel spins results in lowest e-- e-repulsion. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 This video explains how to use the Aufbau principle and a diagonal diagram to write electron configurations. The electron configuration of Nitrogen 7N will have 2 electrons in the innermost shell 1s and 5 electrons in the next shell which is divided into two subshells 2s and 2p.
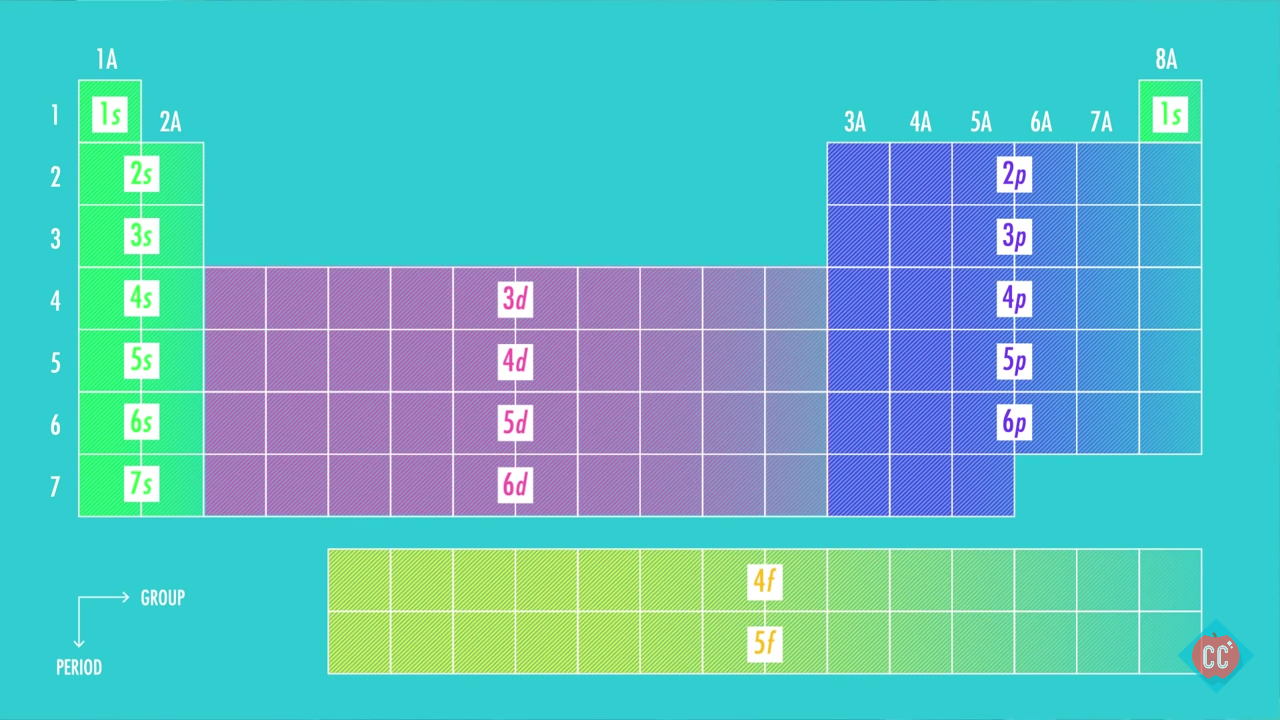
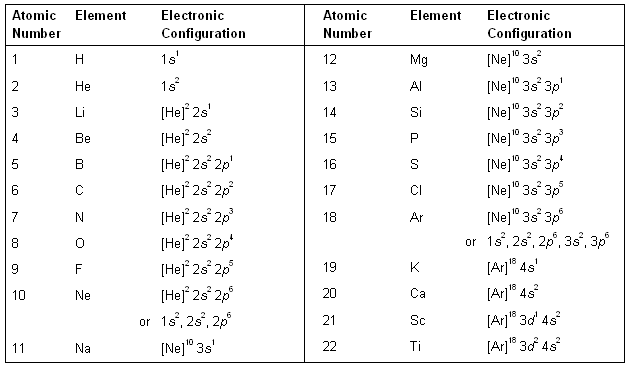
This article provides you with an electronic configuration chart for all these elements. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. Electronic configuration of the neutral Iodine atom.
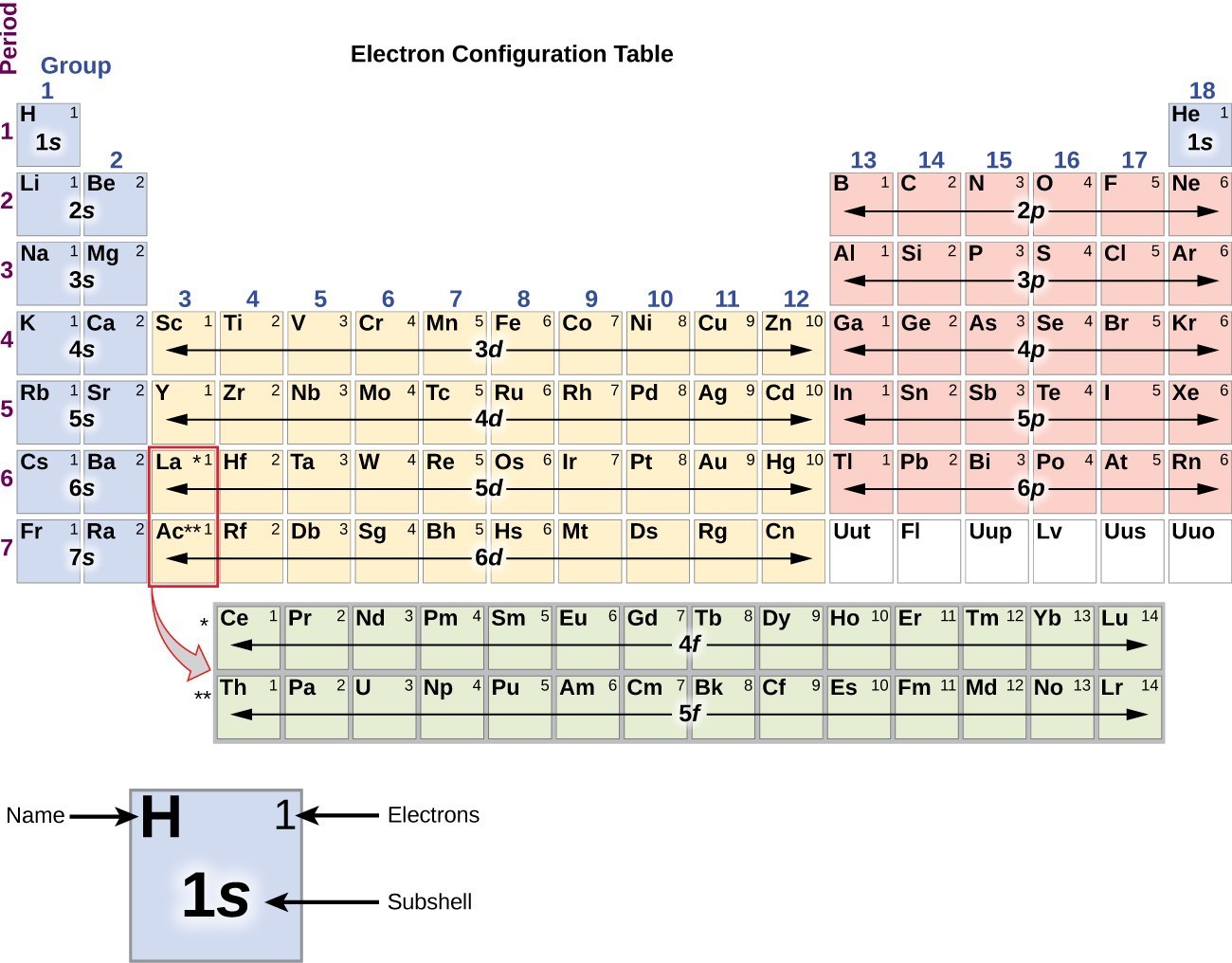
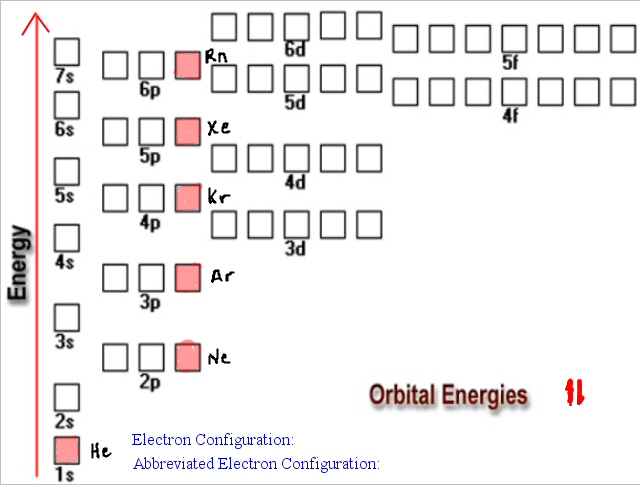
Electron Configuration Chart Electron Configuration of all the elements in table chart Element Atomic Number Element Symbol Element Electron Configuration 63 Eu Xe 4f7 6s2 64 Gd Xe 4f7 5d1 6s2 65 Tb Xe 4f9 6s2 66 Dy Xe 4f10 6s2. Higher the value of nl for the orbital higher is the energy. Weve all seen and use the so-called Aufbau Diagram Figure 1.
Its electron configuration is. BUT what we havent. Here are a number of highest rated Electron Configuration On Periodic Table pictures upon internet.
What is the ground state electron configuration for Element 40. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵ Still since 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ is the electronic configuration of the last previous noble gas. We identified it from trustworthy source.